FlashRNA™ : safe RNA delivery
Even if the risk of adverse side effects is very low with third generation lentiviral vectors, their integration site is unpredictable and can potentially lead to undesirable side effects, such as oncogenesis.
RNA delivery has thus become a safer option when stable and long-term expression of the gene of interest is not needed. LentiFlash® keeps only the advantageous properties of lentiviral vectors to enter target cells (transduction) and RNA transfer without any original viral sequence nor its integrative character.
It makes it possible to envisage therapeutic approaches based on the expression of antigens or antibodies, on genome editing or for regenerative medicine and reprogramming.
Gene editing
The CRISPR technology is revolutionizing the world of pharmaceutical research in many applications such as hereditary diseases, oncology or infectious diseases.
However, for optimal use, this technology must be carried using an efficient delivery system, which implies:
- A high penetration rate into target cells
- A high level of editing of the targeted gene
- The preservation of the viability and the original cell phenotype
- An all-in-one delivery system (ex: sgRNA guide(s) + Cas9)
FlashRNA™, as a RNA delivery system is particularly suitable to carry CRISPR-Cas9 into any cell type while preserving their original cell phenotype and viability and limiting off-target effects, thanks to a fast and transitory nuclease expression.
FlashRNA™ efficiently and safely carrying CRISPR-Cas9 system for cancer immunotherapy using primary T cells
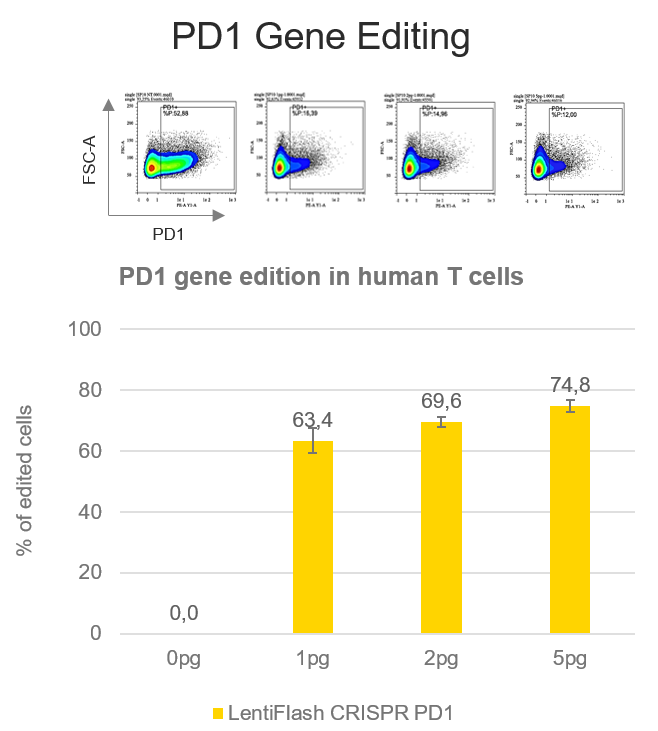
The blockade of PD1/PD-L1 interaction has shown promising results in cancer immunotherapy, overcoming the tumor cells escape to T cells immune response.
A PD1 disruption rate of 74.8% is reached in human primary T cells using FlashRNA™ (formerly LentiFlash®) to deliver a guide RNA targeting PD1 gene and the Cas9 in a single step, at a dose of 5 pg p24 per cell. Thanks to the knock-out of PD1, T cells will not be able to interact with PD-L1 and will be effective against the tumor.he blockade of PD1/PD-L1 interaction has shown promising results in cancer immunotherapy, overcoming the tumor cells escape to T cells immune response.
A PD1 disruption rate of 74.8% is reached in human primary T cells using FlashRNA™ to deliver a guide RNA targeting PD1 gene and the Cas9 in a single step, at a dose of 5 pg p24 per cell. Thanks to the knock-out of PD1, T cells will not be able to interact with PD-L1 and will be effective against the tumor.
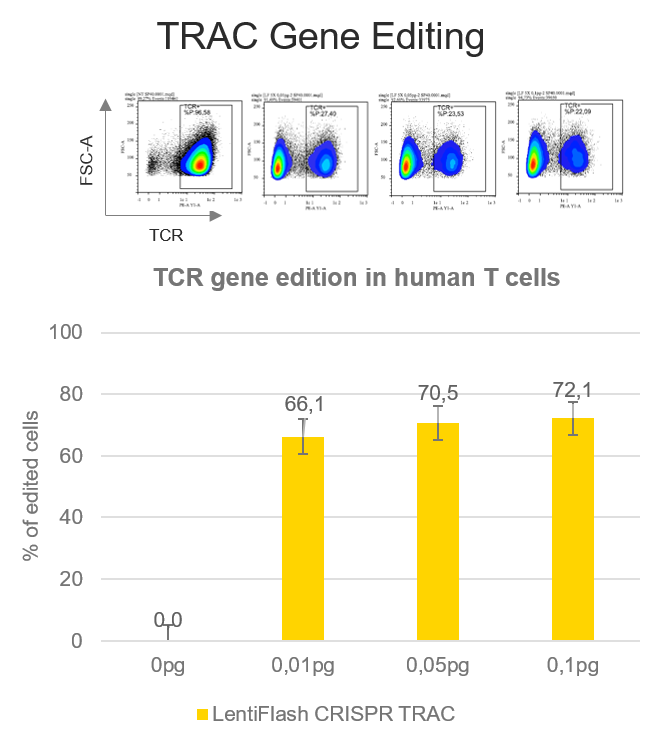
CAR T cells strategies require to knock-down the endogenous T cell receptor (TCR) in order to express a chimeric receptor (CAR) targeting tumoral cells.
A TCR disruption rate of 72.1% is reached in human primary T cells using FlashRNA™ to deliver a guide RNA targeting the alpha chain of the TCR (TRAC gene) and the Cas9 in a single step, at a dose 0.1 pg p24 per cell only. Thanks to the knock-out of the TCR, T cells can be subsequently modified with an integrative lentiviral vector to express a CAR of interest at their surface.
FlashRNA™ delivers efficiently and safely CRISPR/Cas9 system
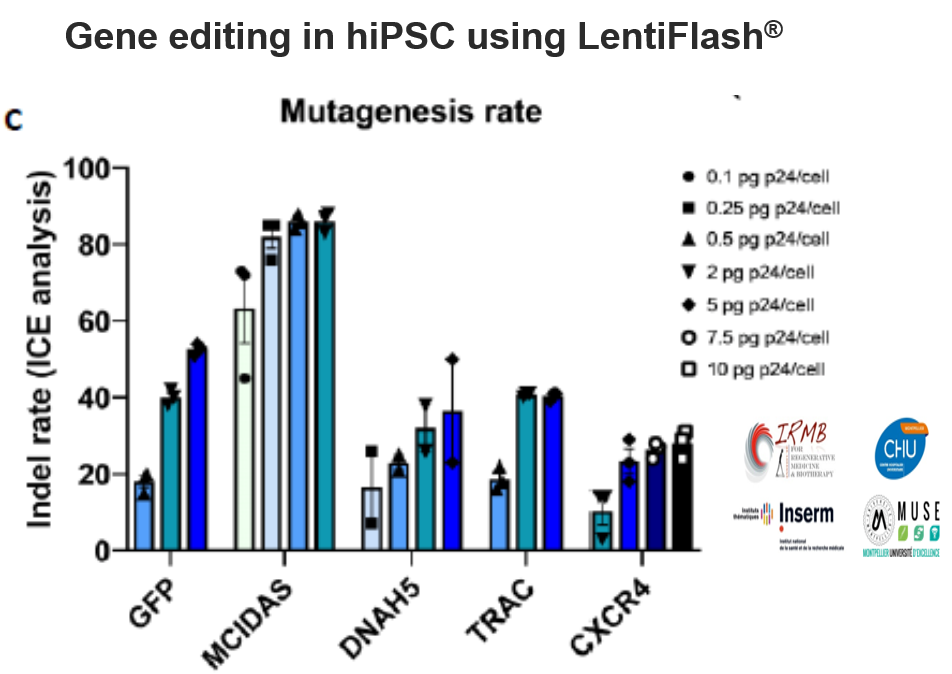
Together, the CRISPR/Cas9 technology and human induced pluripotent stem cells (hiPSC) hold tremendous potential for basic research and cell-based gene therapy. However, the fulfillment of these promises relies on our capacity to efficiently deliver exogenous nucleic acids in these cells.
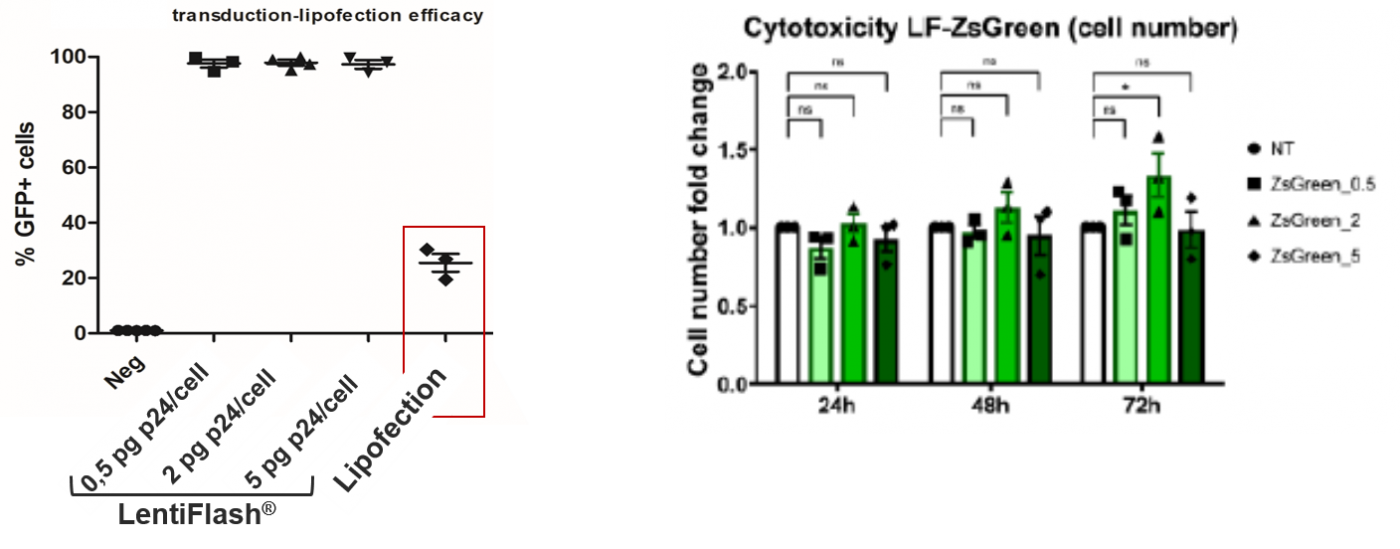
Compared to another RNA delivery approach FlashRNA™ particles deliver RNA without any induced-toxicity:
FlashRNA™ particles transiently deliver in a single step the CRISPR-Cas9 components (Cas9 mRNA and guide RNA) to generate gene knockout with high indel rate (up to 85%) at multiple loci.
Editing efficiency depends on gene accessibility and inherent guide RNA potency.
(Mianné J, et al. BMC Biol. 2022 Jan 7;20(1):8)
Vaccination/immunotherapy
FlashRNA™ particles are a great choice for carrying RNA to transport antigens, nanobodies and immuno-modulators ex vivo and in vivo in order to stimulate the immune defenses of patients.
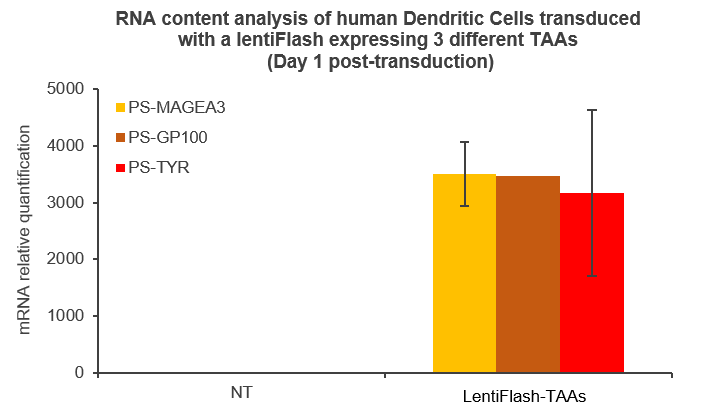
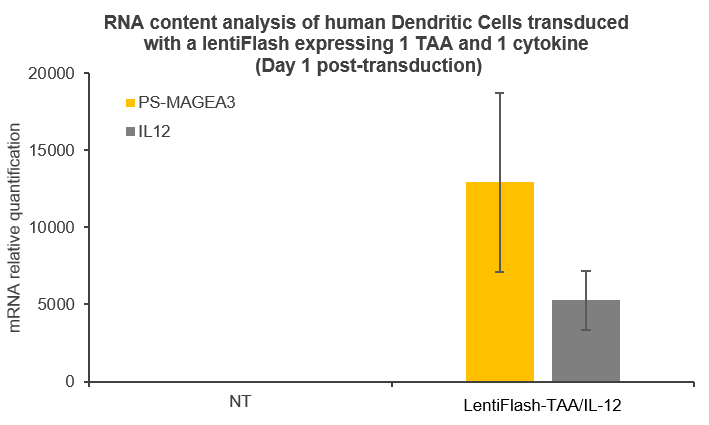
Here are some data showing that FlashRNA™ (formerly LentiFlash) can efficiently deliver several RNA encoding multiple tumoral antigens or immunomodulators in human primary dendritic cells:
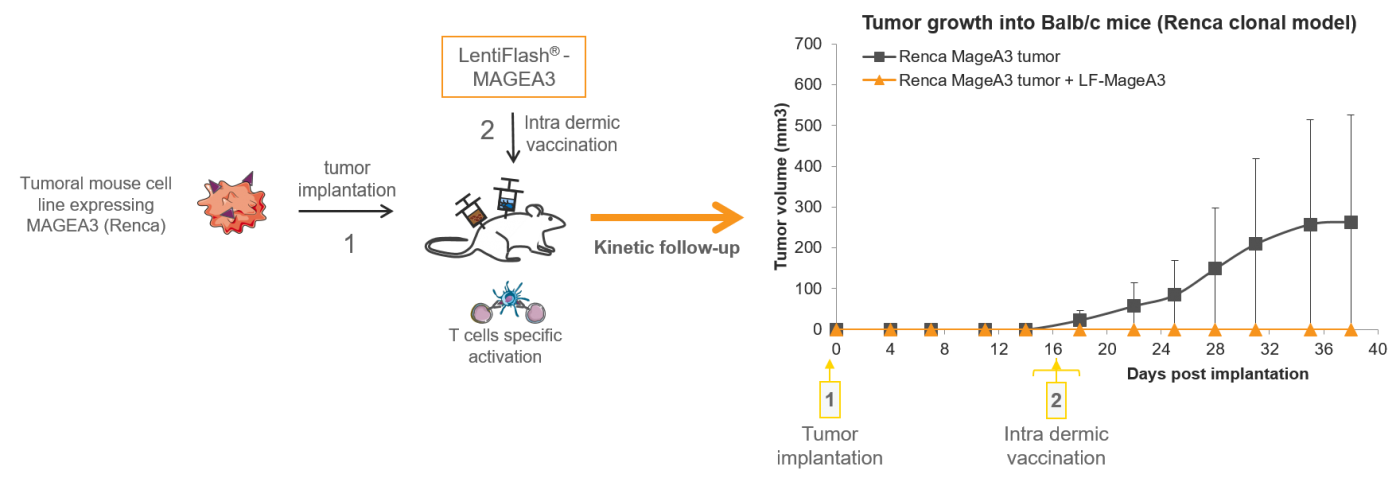
In vivo antigen delivery mediated by FlashRNA™
FlashRNA™ intra dermic delivery of tumoral antigens mediates rejection of progressive tumors (0,5µg p24/dose) in a syngenic tumoral mouse model.
Stem cell /regenerative medicine
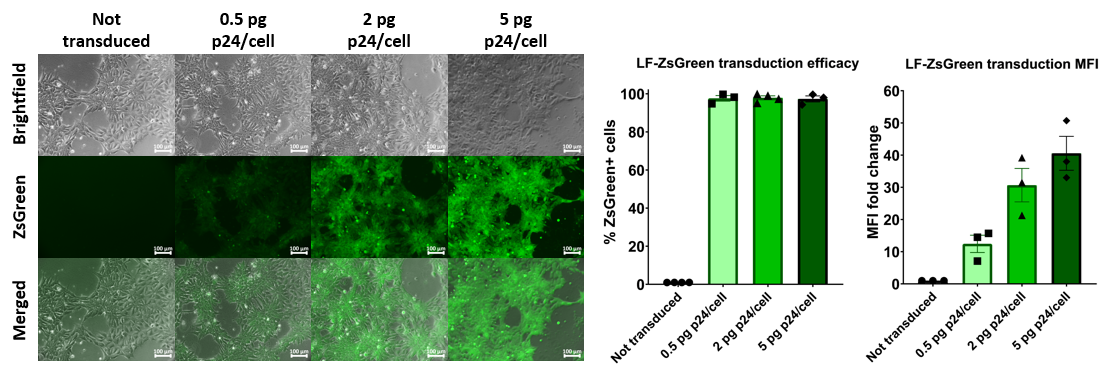
FlashRNA™ efficiently transduces human iPSC cells
In 2009, the first clinical trial approved by the FDA paved the way for the use of human Pluripotent Stem cell (hPSC)-derived cells into the clinic. Since then, several clinical trials have demonstrated the safety of cell therapy products based on hPSC-derivatives.
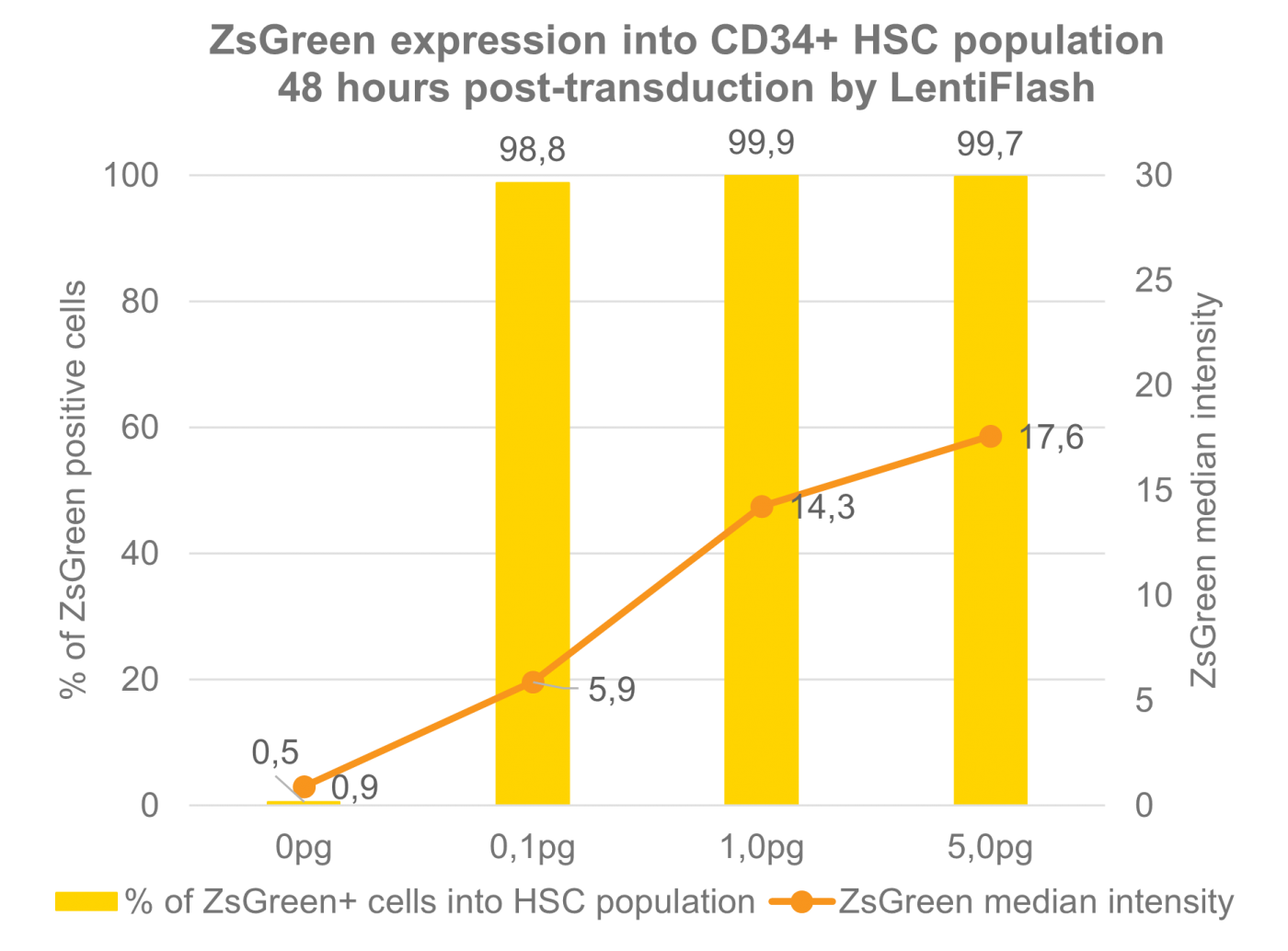
Human induced-pluripotent stem cells transduced with increasing doses of FlashRNA™ (formerly LentiFlash®) show a very high level of transduced cells at low dose and an increasing level of expression when increasing the quantity of particles applied per cell.
FlashRNA™ efficiently transduces human HSC cells
FlashRNA™ can be efficiently used in vivo
It is currently assessed for a gene therapy program aiming to restore lymphatic flow lymphedema in patients treated for breast cancer.
Reprogrammation
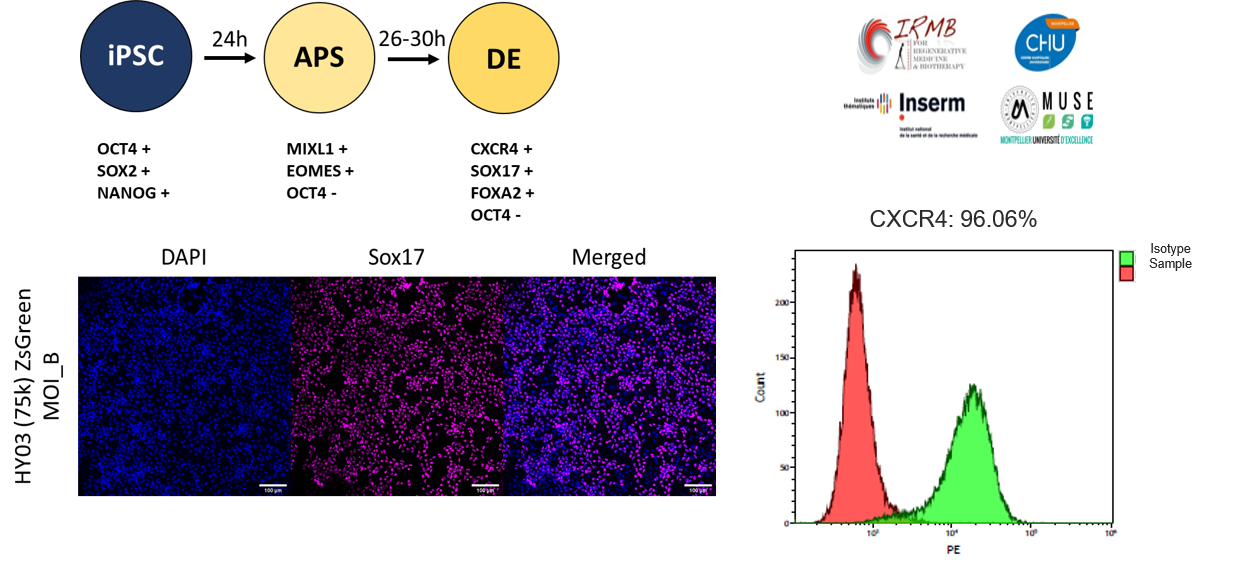
FlashRNA™ tranduces human iPS cells without altering their differentiation ability
FlashRNA™ delivers different RNAs to promote a cell-fate shift in human MSCs
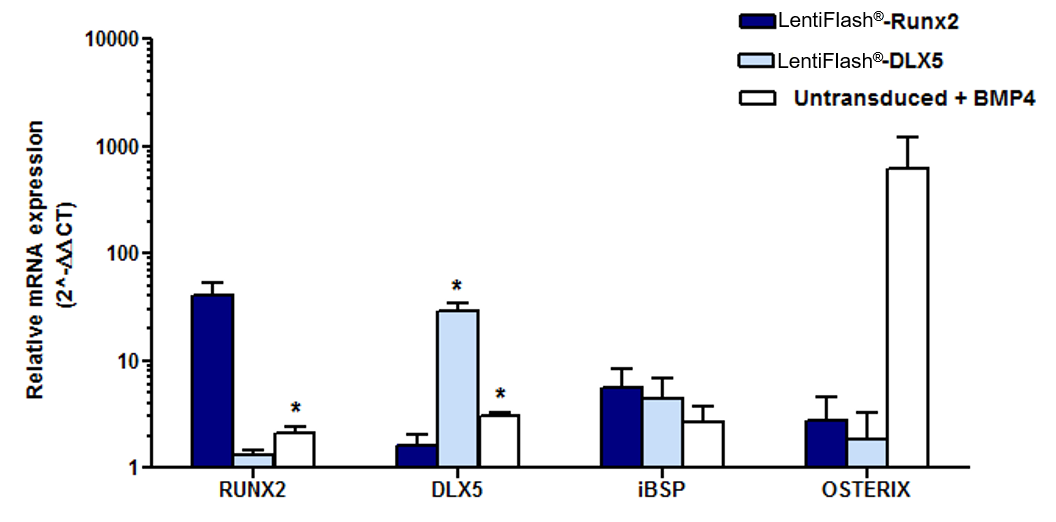
Highly immature primary human Mesenchymal Stem Cell-like cells (MSC-l) were transduced with FlashRNA™ carrying RUNX2 mRNA or DLX5 mRNA (5 pg p24/cell). Controls cells were untransduced and BMP4-treated to differentiate MSC-l into functional osteoblasts.
58 hours post-transduction, Real-Time quantitative PCR genomic analyzes show that the endogenous RUNX2 and DLX5 mRNA levels significantly increased in transduced MSC-l cells. In addition, the osteoblastic marker integrin-bone sialo-protein (iBSP) is upregulated whereas the osteoprogenitor master factor OSTERIX showed no change in expression, suggesting a commitment of MSC-l towards an early osteoblastic lineage stage. (Prel et al. Mol.Ther. 2015)
Innovations
We are committed to constantly innovating in order to improve the properties of our vectors and make them the best tool for your project. Our team of experts works on:
- Improving the duration of gene expression provided by RNA delivery
- Modifying particles pseudotype to target specific cell types
- Optimizing production protocols to ensure any cassette, even very complex polycistronic ones can produce at a high titer, with a high infectivity.