Our experts for your project success
Along with providing high quality lentiviral vector products, Flash Therapeutics offers its expertise and support to clients in areas such as project management, scientific design, bioinformatics, cell analyses, and regulatory questions.
According to their needs, clients may tailor Flash Therapeutics level of project intervention, from simply delivering lentiviral vector products to complete client support.
Depending on your application, Flash Therapeutics provides both integrative lentiviral vectors and non-integrative LentiFlash® particles.
Flash Therapeutics provides integrative lentiviral vectors for gene therapy and immunotherapy. A cutting-edge technology, derived from lentiviral vectors but non integrative is named LentiFlash® and has been specifically developped for regenerative medicine, gene editing and vaccination / immunotherapy studies
-
Lentiviral vectors
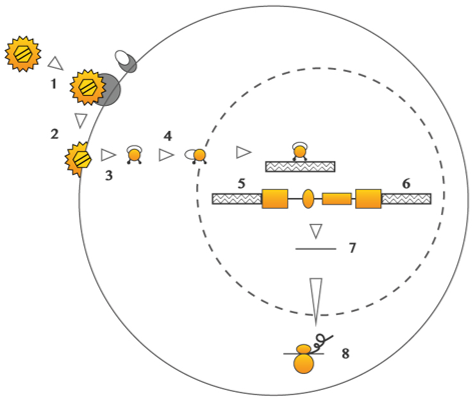
Conventional lentiviral vectors, which lead to DNA integrated into the genome, are a leading delivery method for applications which require a stable and long-term gene expression. -
LentiFlash® particles
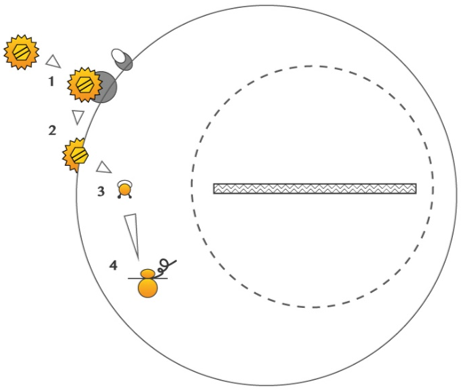
LentiFlash® combines a transient expression with a safe and efficient RNA delivery. This proprietary technology allows the delivery of multiple RNA species, without any genomic scar.
A unique patented purification process
Through a patented process in serum and antibiotic free culture conditions, followed by several steps of concentration and purification, Flash Therapeutics provides lentiviral vector suspensions that are up to 99% free of protein and DNA impurities.
The purification process used by Flash Therapeutics guarantees :
- Conservation of phenotypic profile of the transduced cells
- Cell responses strictly related to the transgene expression
Flash Therapeutics offers two qualities of vector, to answer different needs, depending on your applications or your project phase/status :
- Start quality (>107 IG/ml)
- Premium quality (>109 IG/ml)
Our production and purification methods ensure efficient transfert of genetic material into delicate or hard-to-transfect cells thanks to highly purified lentiviral vector products.
A robust and strong titration method
Flash Therapeutic provides lentiviral vectors titer in IG/ml, which represents the true count of functional particles.
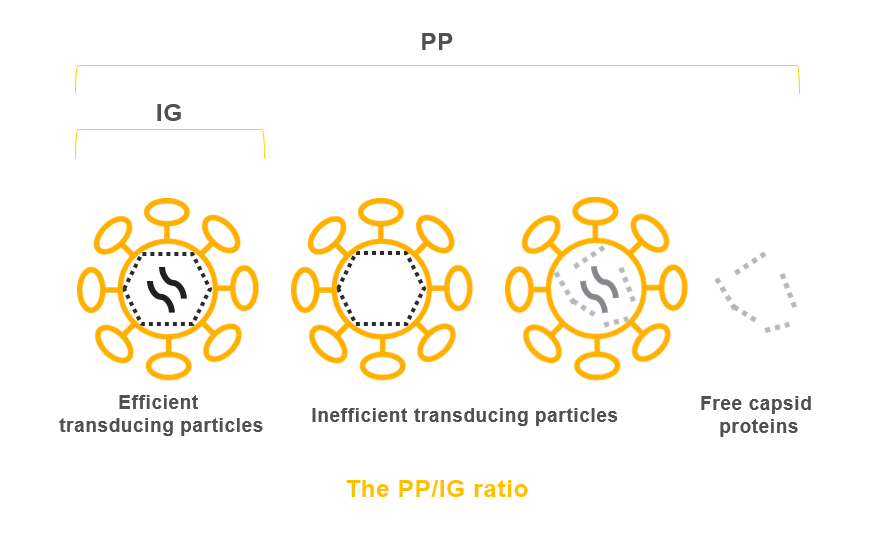
Many of our competitors do not provide titers in IG/ml units but the full count of physical particles (PP), which includes damaged, empty particles and free p24 proteins as shown below.
During the production of viral vectors, functional and non functional particles are produced. A functional and complete particle is called a transduction unit, it is quantified by a robust qPCR method, in Integrated Genome units (IG).
Physical Particles (PP) represent non functional and functional particles, it is quantified by a p24 ELISA assay but it does not allow a precise and reproducible transduction of the target cells.
The PP/IG ratio is a good measure of the quality of the vector produced. Flash Therapeutics offers high quality vectors with a PP/IG ratio decreasing as we progress through our production process and purification steps.
To increase the ratio of functional particles, it is essential to reduce the production of PP in the viral supernatants. Having a reliable and discriminating titration method for the different types of particles is also a key point that Flash Therapeutics offers.
Contact Antoinette to design your project

|
Antoinette Crooke - Global Account Manager
|