10 best practices for a good transduction efficiency
- Lipid or chemical transfection,
- Viral vectors…
A wide range of tools is available for gene transfer experiments. Among these, the lentiviral vector technology has proven to be an effective tool with significant benefits. This is mainly due to its efficiency, its gentleness with delicate cells, the stability of expression and the shortened time lapse of experiments. Unfortunately, results are not always living up to expectations. To prevent failure, several parameters must be taken into account. We describe in this article 10 best practices to follow for a good transduction efficiency.
1- Know your cells
Each cell type, primary or immortalized cells, has particular needs such as specific complete cell culture media, an optimal cell density or concentration for seeding, specific treated microplates or flasks... Knowing and following these needs allows to have cells in optimal condition to perform a good transduction using lentiviral vectors.
2- Check the quality of your lentiviral vector batch
Parameters determining the quality of a lentiviral vector batch are concentration and purity. Be aware that low quality lentiviral vector leads to adverse transduction effect on your cells. That may result in proliferation arrest, loss of cell phenotypes, modifications of cell biomarkers or induction of cell senescence. That's why for primary cells, stem cells or some immortalized cells, we recommend to use highly purified and concentrated lentiviral vectors.
3- Optimize the transgene expression cassette
Several elements have to be taken into consideration when using lentiviral vectors. Depending on your goals you have to consider the following key points:
- The SIN constructs: ΔU3 and the 5' promoter into the 5' LTR
- The promoter that drives your gene of interest can be ubiquitous or cell-specific, constitutive or inducible
- The post-transcriptional Regulatory Elements such as WPRE which allows to increase the transgene expression into the host cells
4- Make sure your titer is in TU/ml
Lentiviral vector batches are provided with a viral titer. Be aware that there exist two types of titer:
The first one is expressed in PP/mL. PP stands for Physical Particles and represents a mix of efficient particles, inefficient transducing particles and free capsids protein leading to an overestimated titer. The second one is expressed in TU/mL. TU stands for Transduction Unit and represents only the efficient and complete particles. The lentiviral vectors titer varies from a lentivector provider to another. For example Vectalys PP/TU ratios are about 100-250 as opposed to 1000 for some competitors.
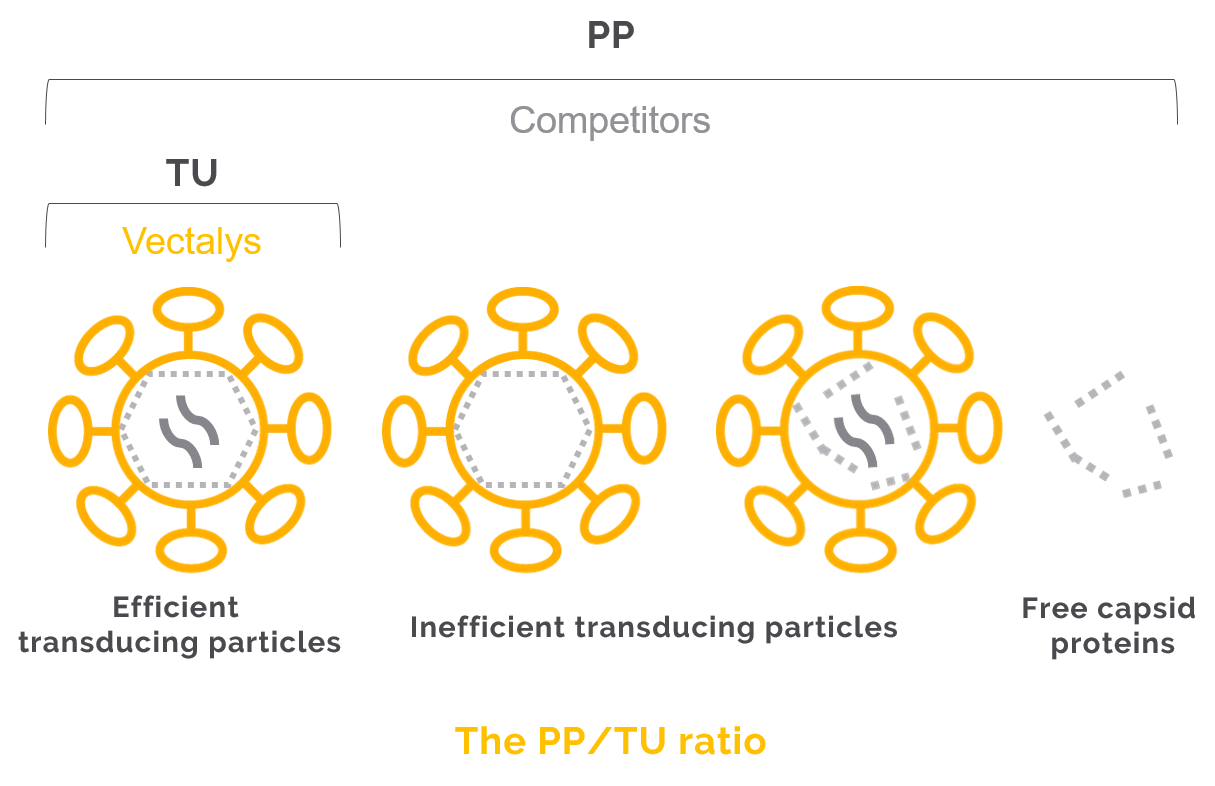
For more information about the determination of such titers, you can download our technical notes for physical particles and transduction unit titration.
5- Follow storage and thawing requirements
Lentiviral vectors batches have to be stored at -80°C upon receipt, and never in liquid nitrogen.
The day of transduction lentiviral vectors have to be thawed differently depending on the aliquot size. For more details about the thawing procedure, check out our FAQ page.
6- Monitor cells sensitivity towards transduction enhancers
To improve transduction efficiency several enhancers are available on the market such as polybrene, protamine sulfate, retronectin, DEAE Dextran. Vectalys recommends the use of polybrene for the most of transduction experiments. Polybrene also known as Hexadimethrine bromide is a cationic polymer. However, cells are more or less sensitive towards this enhancer. In order to determine this sensitivity, you can perform a polybrene toxicity assay by using a concentration range from 0 to 8µg/ml on the cells of interest. If the polybrene is not enough to improve transduction efficiency, it is recommended to test another enhancer such as protamine sulfate or DEAE dextran.
7- Mycoplasma contamination has to be checked
In order to have good and repeatable results, cells have to be in good health. We know that mycoplasma can produce numerous effects in the infected cell cultures leading to altered levels of protein, RNA and DNA synthesis as well as alteration of cellular metabolism. Moreover, these organisms are resistant to most antibiotics commonly employed in cell cultures. Therefore, checking cells for mycoplasma contamination is essential.
8- Identify the optimal M.O.I.
The optimal M.O.I. (Multiplicity Of Infection) corresponds to the number of efficient lentiviral vector you need per cell in order to obtain the maximal percentage of transduced cells.
Each cell type has different permissiveness towards lentiviral vectors. In order to determine this permissiveness as well as to obtain the optimal M.O.I. you can transduce your cells of interest with a range of lentiviral vector expressing a fluorescent reporter (from M.O.I. 0 to 100).
After this transduction assay cells have to be harvested and analyzed by flow cytometry. Thanks to the percentage of transduced cells and the fluorescence intensity, you will be able to select the right M.O.I for your cells of interest.
9- Do not forget the incubation time
In order to efficiently transduce the cells of interest, the transduction mix has to be left on the seeded cells for a minimal amount of time (enhancer + complete cell culture media + viral supernatant).
All lentiviral vectors present in the transduction mix need at least 5 hours to penetrate the cells of interest. Based on the experiment, the transduction can be left from 5 hours to an overnight incubation.
10- Renew the media after incubation
After incubation of cells with the transduction mix, the transduction mix has to be discarded and replaced with fresh complete culture media in order to remove residuals of polybrene or other enhancer.
And one last thing, do not forget to take a look at your cells !
If you need any further advice for the transduction of your cells, our experts would be happy to help.
Share this article:
