Revolutionizing Therapeutics with RNA delivery platform : FlashRNA®

Recent advances in gene and cell therapy highlight the complex challenge of delivering therapeutic agents tailored to specific diseases. While DNA-based therapies have gained notoriety (ILV & AAV administration), the focus is now shifting to RNA-based therapies as a versatile alternative, that can target undruggable pathways(1). RNA therapeutics are a rapidly expanding class of medicines that will change the standard of care for many diseases and bring personalized medicine up to date. It is a disruptive therapeutic technology. This shift is driven by the need for a universal delivery tool that can be used for a wide range of applications while minimising risks, particularly genotoxicity. RNA-based therapies thus offer the flexibility required to target specific therapeutic applications requiring transient expression, whether for gene modification, regenerative medicine or immuno-oncology. This field of application encompasses triggering cellular processes, modifying genetic sequences or directing cells along specific differentiation pathways. Once optimised, these genetically modified cells become effective medicines, illustrating an innovative approach in the landscape of next-generation 2.0 therapies(2).
To overcome the safety limitations of AAV and lentiviral vectors, a revolutionary RNA delivery tool called FlashRNA® (formerly LentiFlash®) has been developed, providing safe and effective solution. This innovative bacteriophage-lentivirus chimera combines the RNA packaging system of bacteriophages with the structure of lentiviral particles, delivering several species of RNA in a single FlashRNA® particle(3). This not only protects the RNAs from degradation but also enables them to be delivered efficiently into the cell cytoplasm, thanks to VSV-G pseudotyping that ensures broad tropism or alternatively use of particular pseudotyping to target specific cell types. RNA bioavailability and protein translation is fast, without any risk of integration into the host genome. As a result, transduction by FlashRNA® does not result in GMO generation. Furthermore, the packaged RNAs are devoid of any lentiviral sequence and are from biological origin: indeed, as they are synthesized in human producer cells, they undergo all cellular post-transcriptional modifications, thus preventing adverse immune responses upon delivery into target cells.
To highlight the three innovative therapeutic strategies - gene editing, regenerative medicine and immuno-oncology - we invite you to discover concrete examples that illustrate each of these approaches.
GENE EDITING
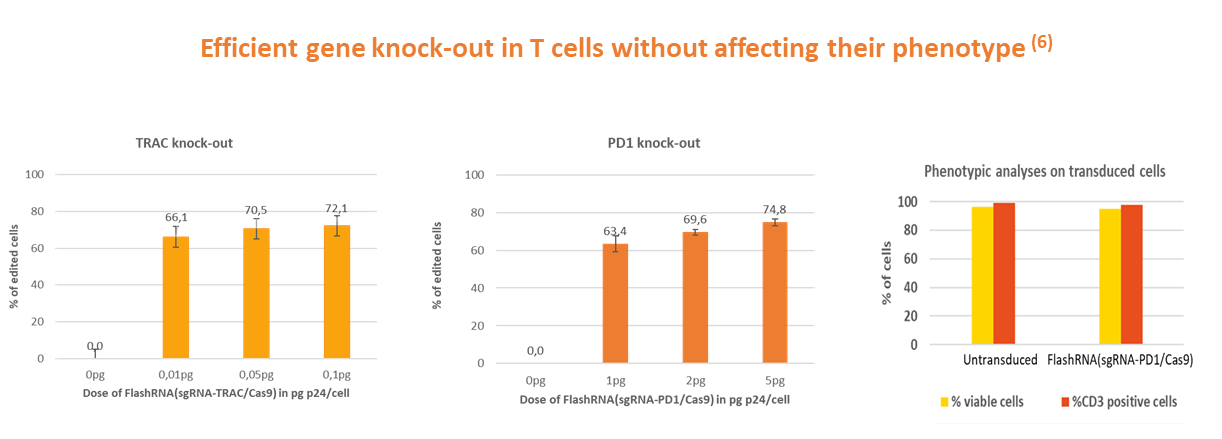
FlashRNA® delivers the CRISPR-Cas9 machinery in a single step in many types of primary cells, from stem cells to immune cells, as well as human induced pluripotent stem cells (iPSCs). This approach guarantees efficient gene editing without compromising cell viability or the differentiation capacity of stem cells. FlashRNA® was successfully used to generate knock-out and gene conversion in iPSCs in vitro (4) as well as to perform base editing in vivo (5).
REGENERATIVE THERAPY
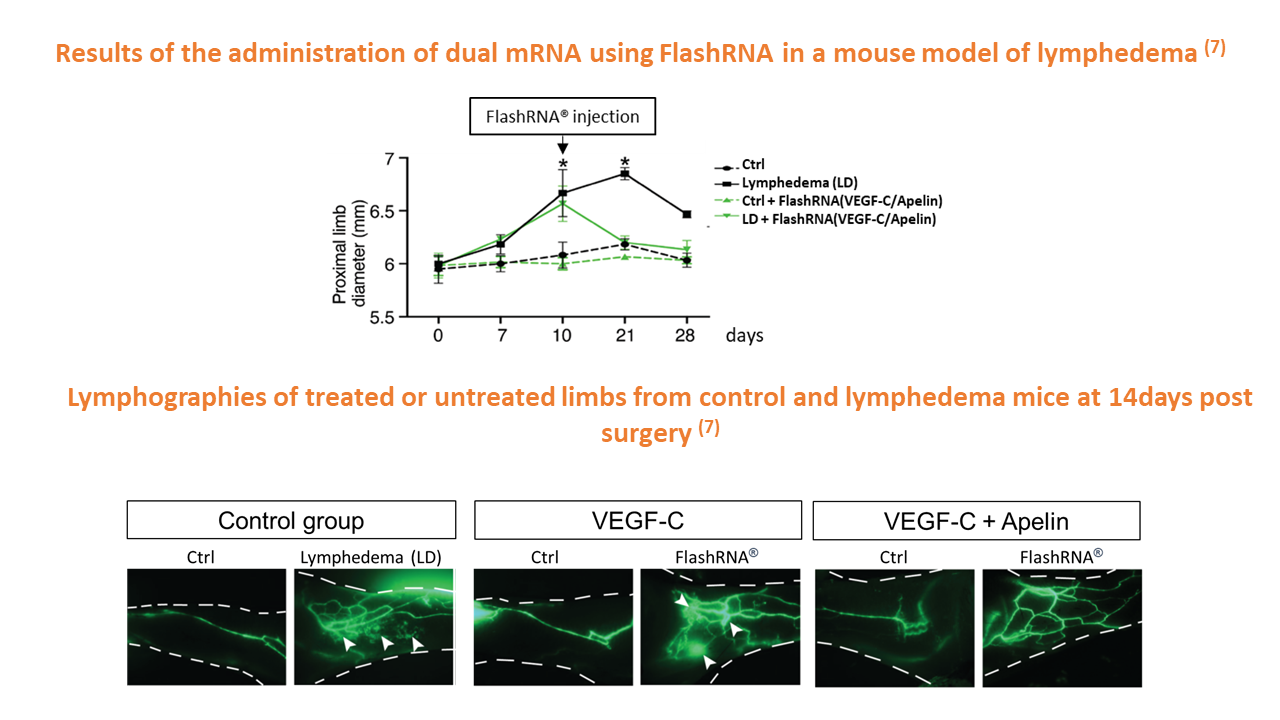
Within the current Theralymph clinical project, FlashRNA® is being used to express two different mRNAs to restore lymphatic function in patients suffering from secondary lymphedema. Preclinical results obtained in mouse models demonstrated the potential to abolish lymphedema when 2 mRNAs are co-delivered, enabling lymphatic flow to be restored. This paves the way for a Phase I/II clinical trial, that will be conduced next year at the Toulouse University Hospital, France.
IMMUNO-ONCOLOGY
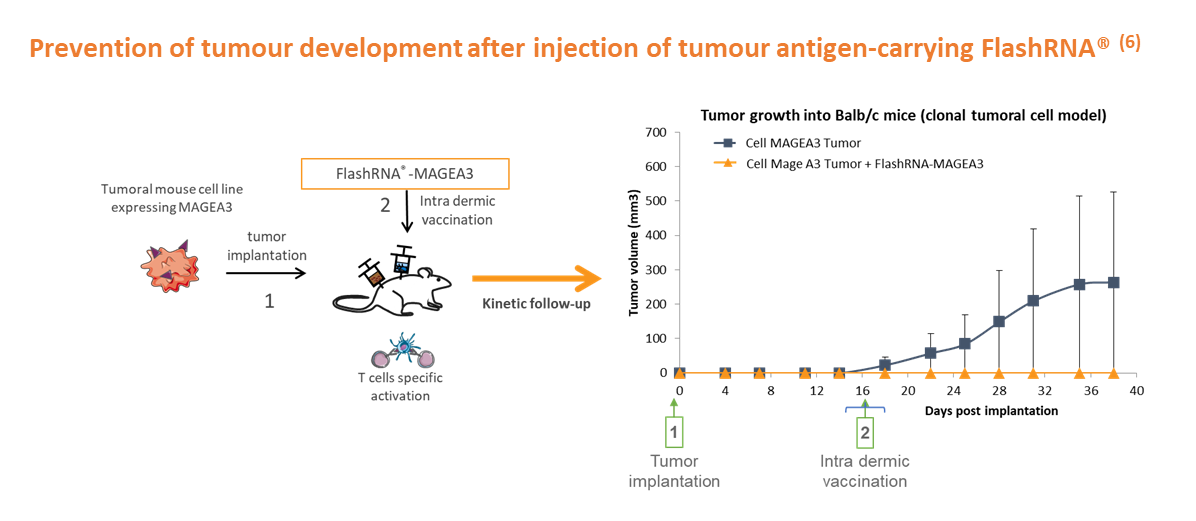
Through direct in vivo delivery of tumour antigen-carrying FlashRNA® or FlashRNA® modified-Dendritic cells, the immune system can then efficiently block tumour development in wild-type mice. This method allows the expression of multiple antigens, enhancing specific immune responses and minimising the risk of tumour relapse.
FlashRNA® is a non-integrative viral RNA delivery system allowing transient, efficient and safe gene expression which marks a significant milestone in the field of gene and cell therapy. This valuable technology is demonstrated by its non-toxic, non-immunogenic nature, combined with a high uptake due to its viral characteristics and its possibility to deliver multiple distinct RNAs. Its versatility, coupled with a production platform compliant with current cGMPs, opens new avenues for therapeutic approaches, providing additional safety considerations compared to other methods.
|
|

|
Alexandra ICHE Gene Engineering Project Manager Flash Therapeutics expert since 2006 |
Let's talk about it:
2. Landhuis, E. The Definition of Gene Therapy Has Changed. Nature (2021) doi:10.1038/d41586-021-02736-8.
3. Prel, A. et al. Highly efficient in vitro and in vivo delivery of functional RNAs using new versatile MS2-chimeric retrovirus-like particles. Mol Ther Methods Clin Dev 2, 15039 (2015).
4. Mianné, J. et al. CRISPR/Cas9-mediated gene knockout and interallelic gene conversion in human induced pluripotent stem cells using non-integrative bacteriophage-chimeric retrovirus-like particles. BMC Biol 20, 8 (2022).
5. Whisenant, D. et al. Transient expression of an adenine base editor corrects the Hutchinson-Gilford progeria syndrome mutation and improves the skin phenotype in mice. Nat Commun 13, 3068 (2022).
6. Flash Therapeutics internal documentation
7. Lamaa, A. et al. Apelin-VEGF-C mRNA delivery as therapeutic for the treatment of secondary lymphedema. 2023.01.05.522869 Preprint at https://doi.org/10.1101/2023.01.05.522869 (2023).