AAV or lentiviral vectors? What should I use for my in vivo experiments?
Chances are that you will never be sure to choose the best serotype for your cells of interest because different labs can use different types, according to the tissue they target but also according to a specific cell type or just because they are used to work with this one... (1, 2) On the contrary, lentiviral vectors (LVs) are VSV-G pseudotyped, which allow them to efficiently transduce any cells, from any species.
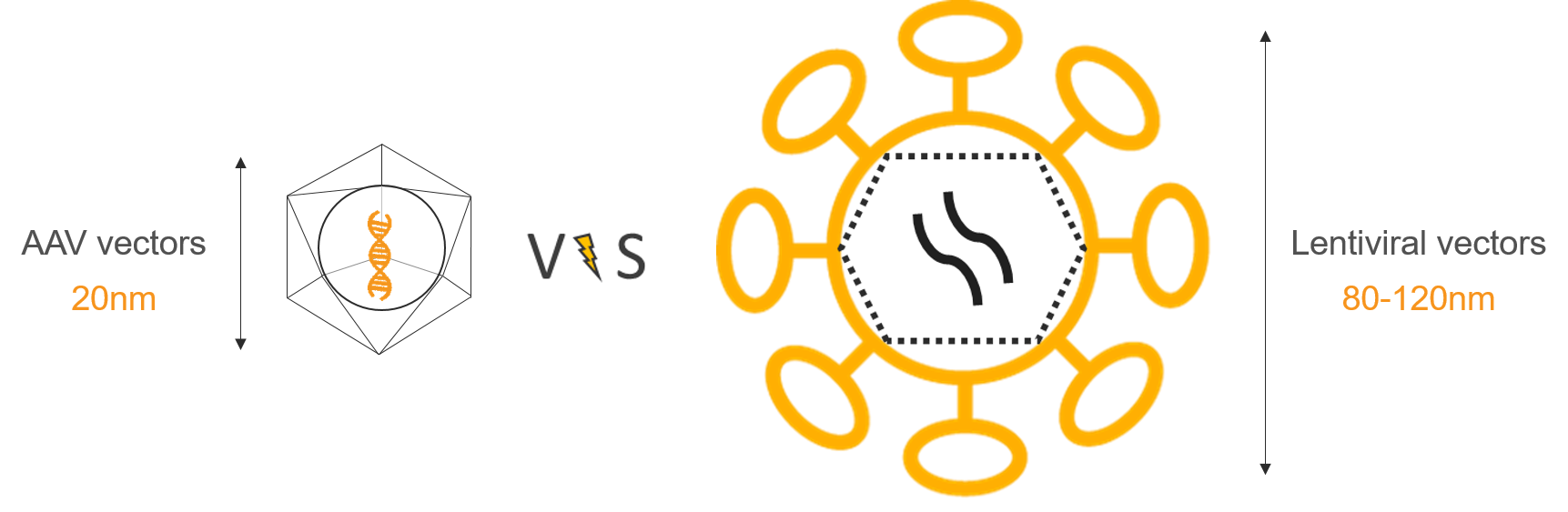
Another point is that AAVs will not integrate the target cell genome as it stays as an episome within the cells, while LVs are integrative and provide a very stable expression. AAVs are smaller particles than LVs, which gives them advantage on their spreading efficiency within particular tissues. But this benefit also brings a major drawback limiting the size of the expression cassette to 4,5 kb max whereas a lentiviral vector can carry a 10kb insert.
Both AAVs and LVs are efficient tools for gene transfer: they can stably induce gene expression in quiescent or dividing cells and they usually do not lead to any immune response into the host. However you have to consider the purity of any vector you use to avoid inflammatory response (3) and to preserve the original cell phenotype and viability after transduction.
If you need to target a cell population specifically without affecting other ones in the same tissue, then your new best friend will probably be: the lentiviral vector. The use of a specific promoter for your cells of interest is a smart option, and lentiviruses will then allow you to validate your strategy in vitro before working in vivo. AAVs are not that good at modifying cell cultures, and adding a specific promoter to your construct may just end up designing a large size cassette which an AAV cannot handle (limit of 4.5kb) (4). Click here to learn more about specific promoters.
Finally, if you look for a system as efficient and clean as the LVs but without its integrative properties you can also use non integrative particles like LentiFlash™, which delivers RNAs directly inside the cells, without any integrative event. This can be a clever choice for genome editing or transient modification of cells to be transferred in vivo.
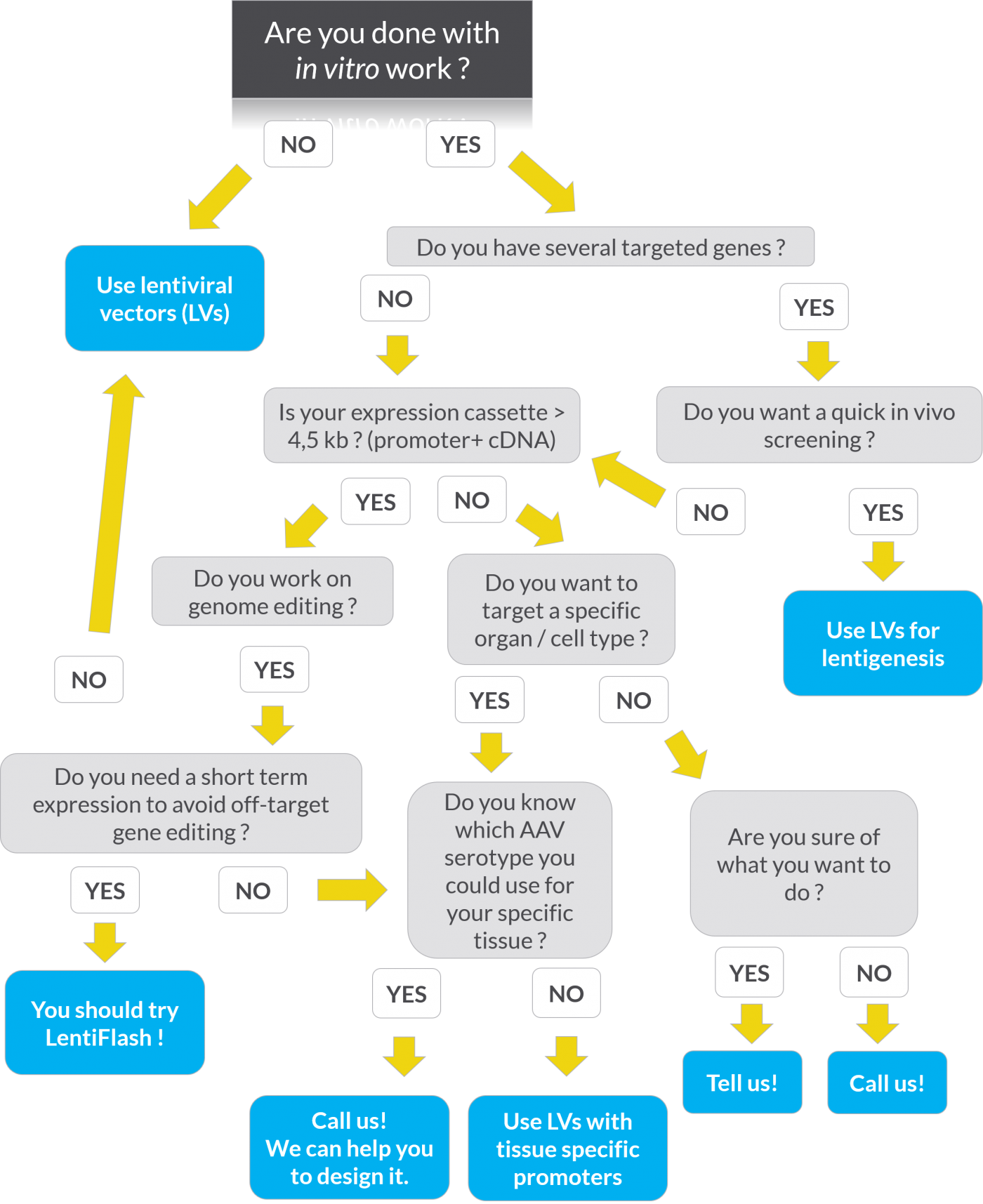
You still do not know which tool you should use? The flow chart below can help you to choose the right delivery tool for your experiments:
References:
(1) Zincarelli, Carmela, et al. "Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection." Molecular Therapy 16.6 (2008): 1073-1080. PubMed PMID: 18414476.
(2) Srivastava A. “In vivo tissue-tropism of adeno-associated viral vectors.” Curr Opin Virol. 2016 Dec;21:75-80. doi: 10.1016/j.coviro.2016.08.003.
(3) Herzog RW. “Complexity of immune responses to AAV transgene products - Example of factor IX.” Cell Immunol. 2017 May 29. pii: S0008-8749(17)30080-1. doi: 10.1016/j.cellimm.2017.05.006.
(4) Grieger, Joshua C., and Richard J. Samulski. "Packaging capacity of adeno-associated virus serotypes: impact of larger genomes on infectivity and postentry steps." Journal of virology 79.15 (2005): 9933-9944. PubMed PMID: 16014954. PubMed Central PMCID: PMC1181570.

|
Christine Duthoit Project leader Cell engineering & Immunology Flash Therapeutics expert since 2009 |