Expertise at your service
-

Experience
High expertise with more than 17 years in lentivirus only & IND deposition -

Potency
High potency & high purity with GMP standards -

GMP & Safety
Quality control plan adapted to your needs for Safety concerns -

support
Increased production capacities with experts dedicated to your project
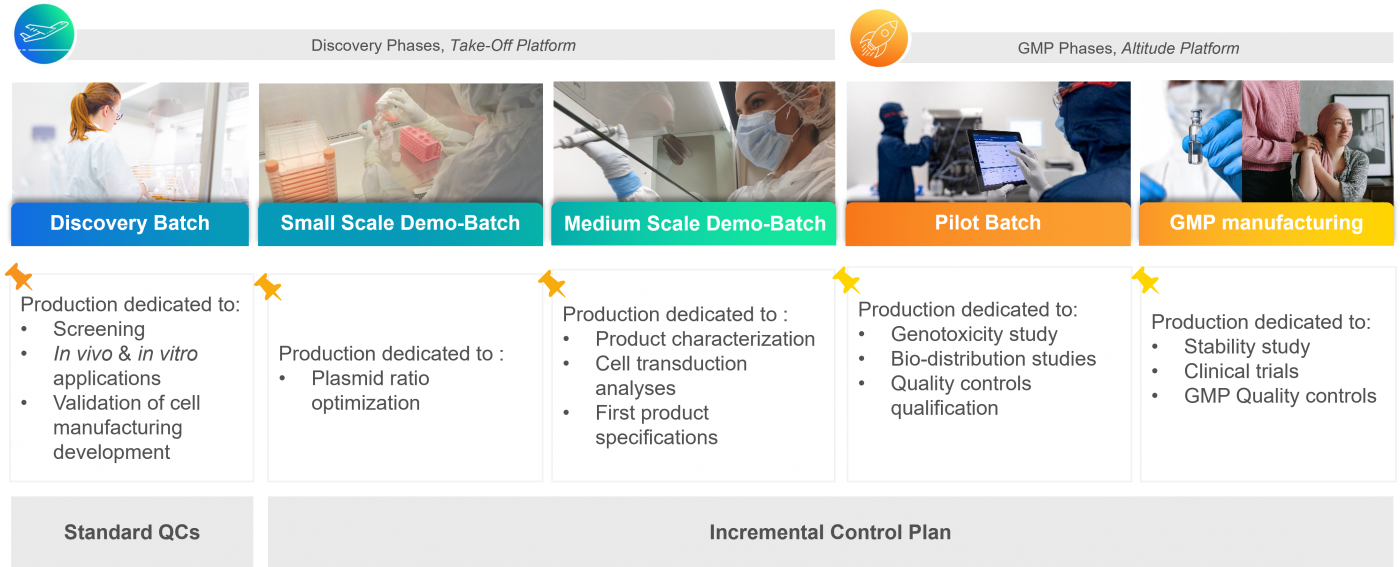
A tailor-made continuum from discovery to therapy
Flash Therapeutics proposes a continuum designed to coincide with the different phases of your clinical trial and regulatory requirements.
Thanks to our patented production and purification process, which remains exactly the same for both R&D-grade and GMP-grade lentiviral vectors, we guarantee the same high quality vectors from your discovery phase to your clinical phase, thereby ensuring the success of your clinical study.
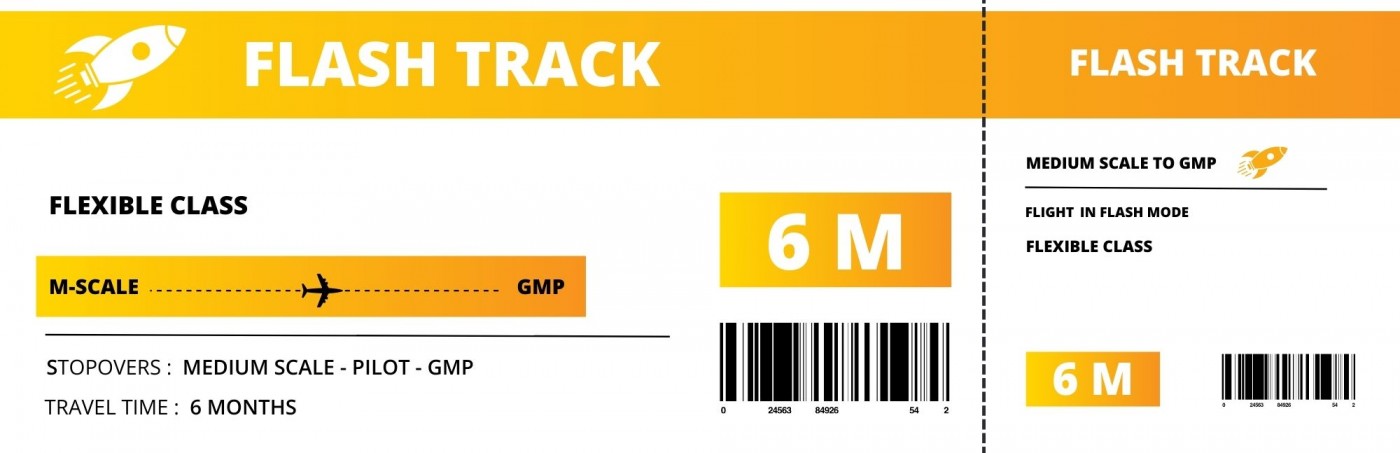
In a hurry ? Discover our time-saving Flash Track
Enter fast into clinical phases and benefit from the full range of Flash Therapeutics experience, capabilities and services.
What's included in your Continuum?
We support you with regular meetings to discuss milestones, project advancement, results and to define future moves.
- Robustness of our production and purification process
- Reproducibility of results throughout the continuum/scale-up
- High transduction efficiency on any cell type, even hard-to transfect cell
- High availability of experts and project managers to monitor each project
Flash Therapeutics provides robust and phase-dedicated documents for all the manufactured batches of your GMP process.
- Facilities: management of physical parameters (air particles, temperature, humidity, pressure), environmental controls
- Devices qualifications (QI, QO, QP)
- Human Resources: Process validation through MPT (open and closed phases) and individual validation through MFT (process developed with bacteria media instead of all raw materials)
- Raw materials: certificates of analysis, audit of providers
- SOP / MOP: managed by the QMS
A customized Quality Control plan to fit reglementary requirements of your project
Our QC plan is validated according to our production process. We perform all standard and specific tests according to your needs. Entrust us with your project, our team will take care of everything.
A dedicated team involved at all steps of the manufacturing process to support our customer.
Flash Therapeutics helps clients determine the best suited lentiviral vector construction for their projects, so that they may obtain up to 100% target cell transduction efficiency.
By organizing regular meetings we allow everyone to understand and contribute to a synergistic workflow:
- Technical meeting ( QCs, transgene expression assay, ...)
- Result meeting QCs plan meeting
- Regulatory support meeting
Contact Antoinette to design your project

|
Antoinette Crooke - Global Account Manager
|
Flash Therapeutics, formerly known as Vectalys, has built its reputation over the last 18 years in lentivirus production. With over 9000 batches produced for 200 customers around the world, FTX's know-how guarantees the success of your projects.
Whether it's R&D, pre-clinical or GMP, we're here to support you.