-
Gene editing
-

Gene Therapy
IRIS program: Cell Therapy to treat inherited immune disorders
The IRIS project seeks to establish gene therapy as the reference treatment for replacing partially human leukocyte antigen (HLA) matched hematopoietic stem cell transplantation (HSCT) as the treatment for very severe primary immunodeficiencies (PIDs): type 3 familial hemophagocytic lymphohistiocytosis (FHL-3), and immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome.
Flash Therapeutics plays a dual role in the project:
- As a cGMP lentivirus provider for FHL-3 phase I/II clinical trial, for the production of an ILV batch as a starting material to genetically modify patients’ autologous HSCs.
- As a partner for IPEX, to explore gene addition and gene-editing approaches, in order to gene modify either the whole hematopoietic compartment or only T cells with the most appropriate technology.
Timeline & funding :
The project was launched in December 2019 for a duration of 5 years (December 2024). This project has received € 9.9 million State funding from the French National Research Agency (ANR) Investments for the Future program (PIA) under grant agreement No. ANR-18-RHUS-0003.
Objectives & technical challenges :
IPEX syndrome is characterized by the specific absence of regulatory T cells. The aim to cure this disease is to try to set up a new curative treatment for patients without resorting to allogeneic HSCT or long-term immunosuppression - both of which are extremely toxic.
Three main tasks constitute this part of the project :
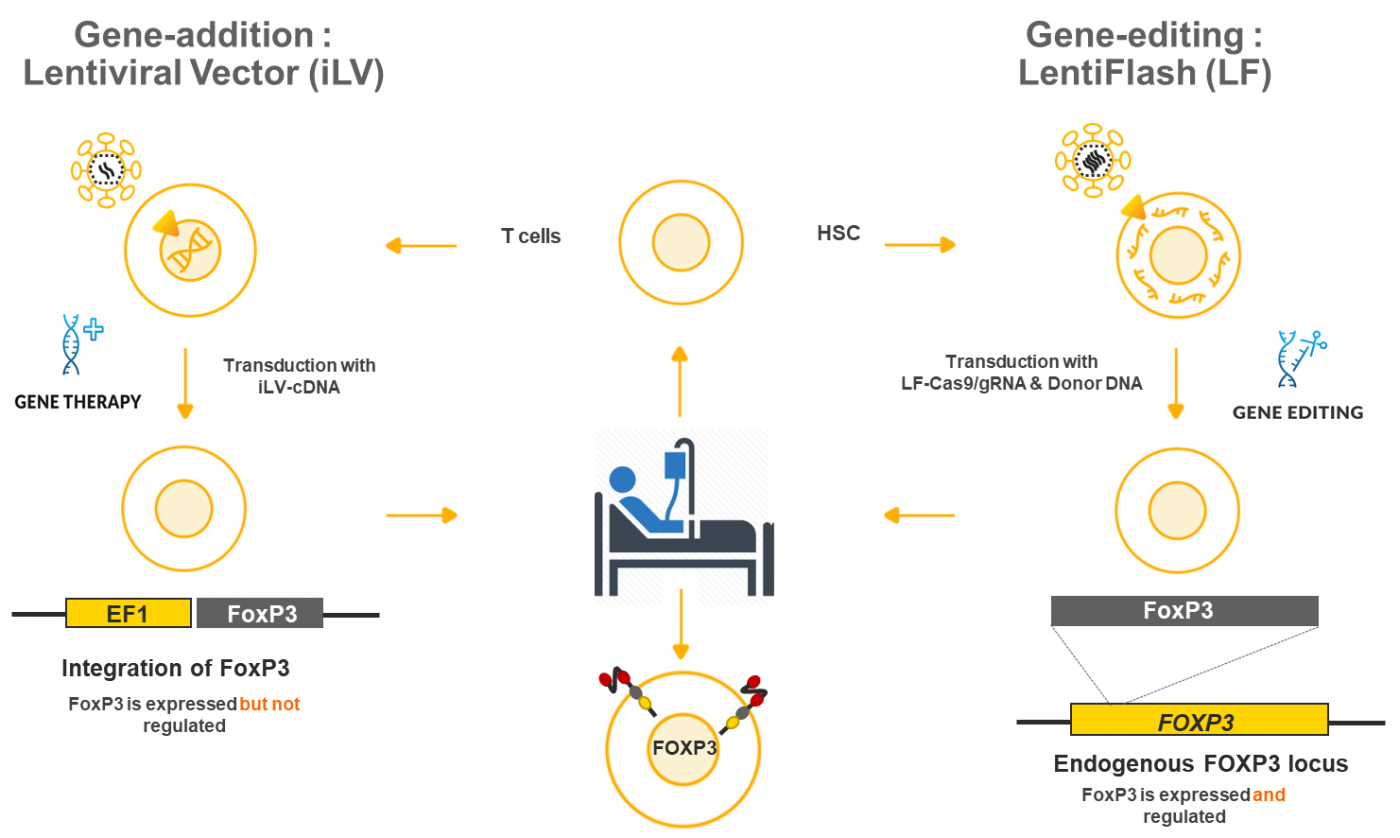
- Treat patients with a gene therapy approach using an integrative lentiviral vector (iLV) that will add normal version of the FoxP3 gene in the targeted cells
- Perform preclinical studies for the gene-editing approach by using a non-integrating LentiFlash® technology to repair the defective FoxP3 gene in the target cells
- Compare safety and efficacy results for the two technologies
Objectives of the gene addition strategy:
- Establish the efficacy of the gene-modified autologous CD4 T cells in a knock-out mouse model
- Conduct a genotoxicity study
- Generate of clinical-grade vector for the expression of FOXP3
- Initiate the clinical trial and the inclusion of patients
Objectives of the gene-editing strategy :
- Characterize in vivo regulatory T cells generated by FOXP3 knock-in in a murine model
- Characterize in vitro of regulatory T cells generated by FOXP3 gene-editing in IPEX T cells
- Characterize the clinical-grade vector
- Conduct safety, biodistribution/persistence and tumorigenicity studies
IRIS will generate intellectual property on vectors, murine models, transduction procedures, results of in vitro and in vivo comparisons of gene addition vs. gene-editing approaches, and the first clinical results from the gene addition trial.
For more information, please download the IRIS project press release .
Consortium Members
Selected by an international committee of experts, this project is led by Pr. Marina CAVAZZANA, and the coordinating institution is Assistance Publique-Hôpitaux de Paris (AP-HP). The selected consortium includes AP-HP, Imagine Institute, INSERM, CERBM/Clinical Institute of Mice, and Flash Therapeutics.
AP-HP
Assistance Publique – Hôpitaux de Paris (AP-HP) is the coordinator of this RHU. It has great expertise in conducting gene therapy trials thanks to its Cell and Gene Therapy Laboratory (LTCG) and the Clinical Investigation Centre/ CIC/Clinical Research Unit (URC).
The AP-HP is bringing several clinical departments (within three different university hospitals) to the IRIS project, from which the Necker Children’s Hospital, and the Department of Biotherapy which specializes in the management of Phase I/II clinical trials in the field of hematopoietic disorders, and will be responsible for the regulatory and ethics aspects of the Phase I/II clinical trials.

IMAGINE Fundation
Established in 2007 as a scientific cooperation foundation by the AP-HP, INSERM, the Université Paris Descartes, the French Muscular Dystrophy Association (AFM), the Fondation Hôpitaux de Paris-Hôpitaux de France and the Mairie de Paris, the Imagine Institute of Genetic Diseases was accredited with university hospital institute status in 2011 under France's major “Investments for the Future” program. The Imagine Institute’s missions – patient-focused research, innovative care, education, training, and technology transfer – have the common goal of establishing better treatment and innovation in care pathways for patients with genetic diseases.
The Imagine Institute and the Innovative Therapies Unit from AP-HP have long-standing expertise in the preclinical development of gene therapy and they have developed a very efficient process for responding rapidly to unexpected clinical results requiring modification of the gene therapy procedures

INSERM U951
U951 is located at Genethon as part of the “integrated genetic approaches in therapeutic discoveries for rare genetic diseases” research unit (UMR_S951). This unit develops proof-of-concept gene therapy for various monogenic diseases (including PIDs). The unit’s bioinformatics group has developed a wet-laboratory protocol and bioinformatics pipeline for rHIV-1 lentiviral vector insertion site analysis (VISA) in the genome of human or murine target cells. VISA should now be developed in terms of quality assurance and compliance for use in early-phase clinical trials.

CERBM (European Medecine and Biology Research Center) / ICS
Phenomin-ICS is a French center of excellence for translational research and functional genomics. It provides a comprehensive set of specialized services to academic and industrial users, and is a major player in European post-genomics programs. It generates and validates mutant mouse and rat models “à la carte”, and performs phenotypic analysis and preclinical studies covering the major physiological systems in order to better understand gene function and human diseases.