Integrative lentiviral vectors can be used in several application domains
Lentiviral vectors are now widely and successfully used for cell therapy approaches thanks to inherent properties that are advantageous over other gene transfer agents, notably their ability to transduce dividing and non-dividing cells, modifying their genome permanently and allowing stable and long-term expression of the transgene of interest.
Immunotherapy
Lentiviral vectors are very efficient to engineer Hematopoietic Stem Cell (HSCs) and immune cells (lymphocytes, NK cells). They are used in therapy since 2003 and are now an essential starting material for cell therapy, as proven by success in B cell leukemias using T cell modified for the stable expression of chimeric expressing receptors (CARs) specific for CD19, CD22 and now other cell specific surface markers. They are also used to treat monogenic gene disorders, thanks to the modification of human HSCs.
Flash Therapeutics can provide you with specific transduction protocols on demand, please contact us.
Flash Therapeutics’ highly pure lentiviral vectors allow to genetically engineer any cell types while maintaining the original phenotype in order to mimic, as much as possible, natural in vivo conditions.
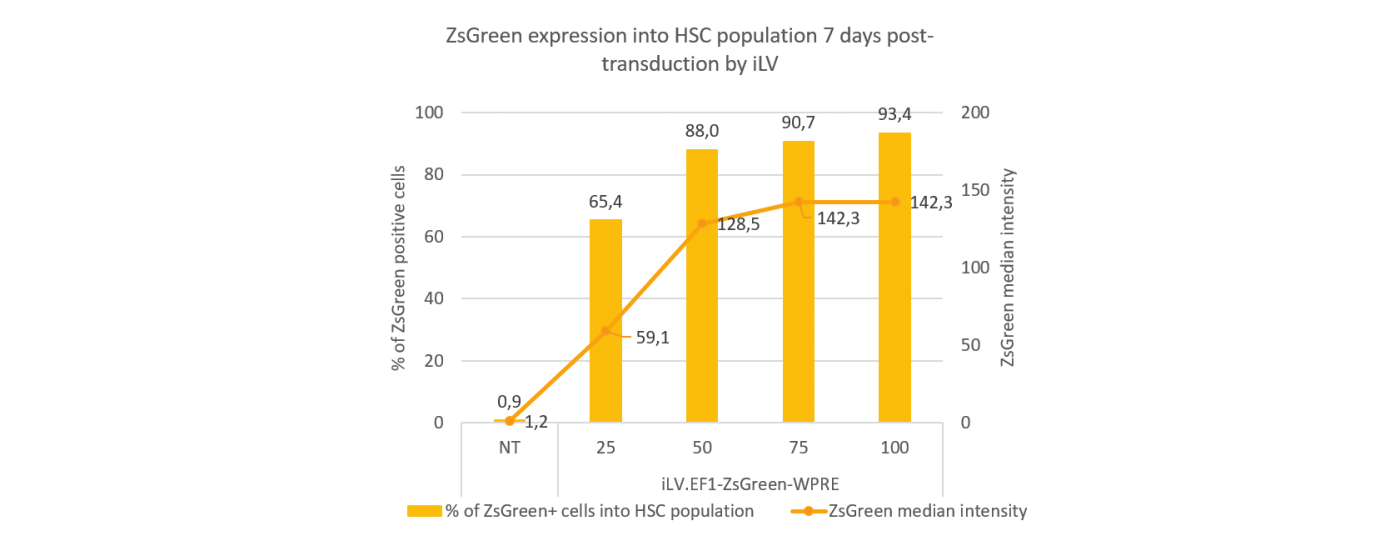
High transgene expression on human Hematopoietic Stem Cells
Human Hematopoietic Stem Cells (HSCs) are harvested from cord blood and transduced by a ZsGreen-expressing lentiviral vector using a range of MOI from 0 to 75 in presence of polybrene 4µg/mL for 16h at 37°C, 5% CO2. Seven days later, the % of ZsGreen positive cells and fluorescence intensity, as well as the expression of CD34 marker are analyzed by flow cytometry.
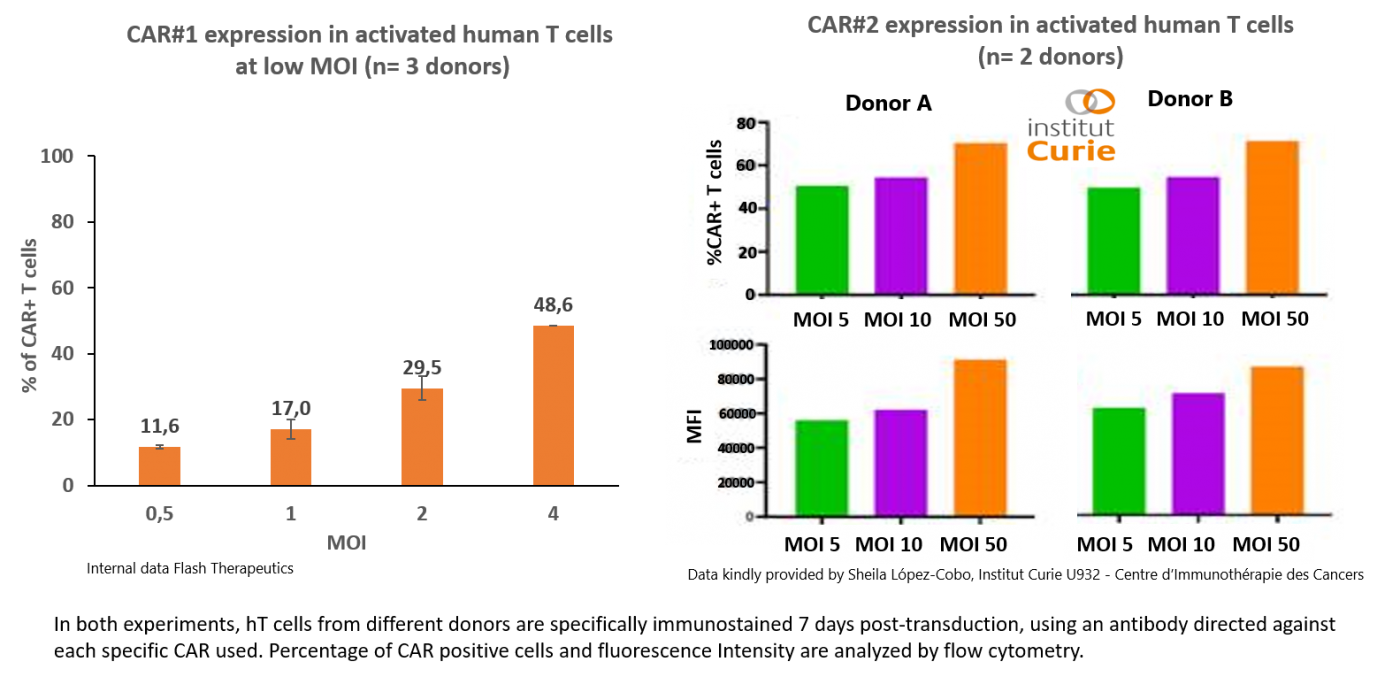
High CAR expression on human T cells
Human activated T lymphocytes harvested from different donors are transduced by 2 different CAR-expressing lentiviral vector using a range of MOI (Multiplicity of Infection) from 0 to 4 (left panel) or 0 to 50 (right panel) in presence of polybrene 4 µg/mL for 16h at 37°C, 5% CO2.
Even at low doses, human primary T cells are efficienty expressing the CAR of interest.
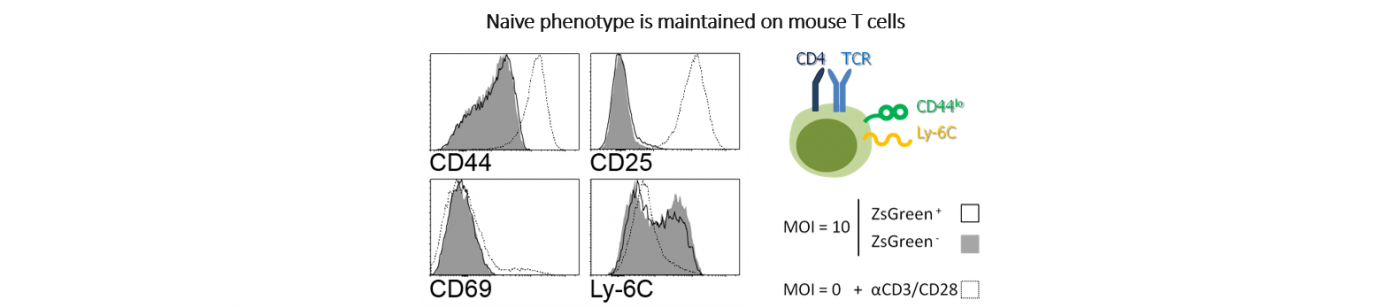
Naive CD4+ T lymphocyte are transduced by a ZsGreen-expressing lentiviral vector at MOI 10 in presence of polybrene 4µg/mL for 5h at 37°C, 5% CO2.
Transduced murine T cells exhibit an original cell phenotype without any changes to T cells specific marker expression such as CD44, CD69, CD25 and Ly-6C, indicating that the use of our highly pure lentiviral vectors does not impact the T cells’ phenotype.
Naive phenotype : low CD44 expression; no CD25 nor CD69 expression which are expressed specifically on stimulated T cells; Ly6 expression.
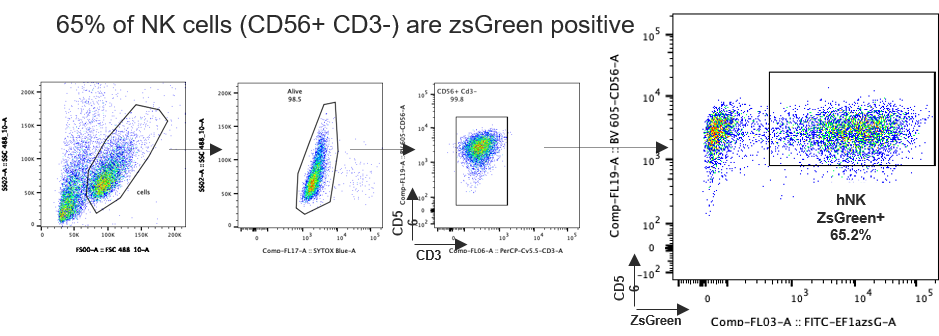
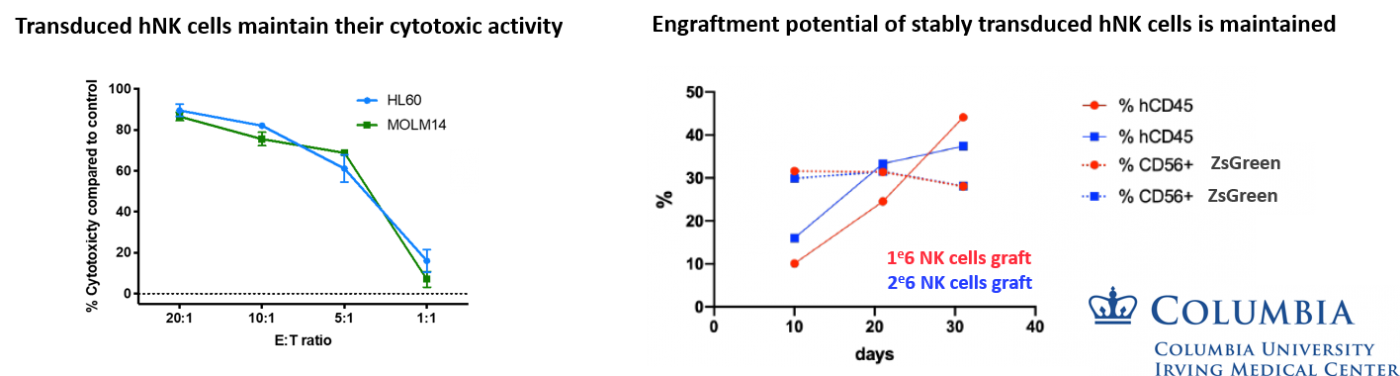
Transduced human NK cells can efficiently kill and be engrafted
hNK CD56+ CD3- Cord blood cells are cultivated using the G-Rex® system (Wilsonwolf), in presence of IL-2, IL-15 and IL-21 and activated with artificial APCs. hNK cells are transduced twice at MOI 50 with a ZsGreen-expressing lentiviral vector. Five days post-transduction, the % of CD56+ CD3- ZsGreen positive cells is analyzed by cytometry.
Transduced hNK cells are fully functional. They can efficiently kill HL60 and MOLM14 target cells in vitro (17h incubation at various Effector: Target cells ratio)
Transduced hNK cells are efficiently engrafted in vivo for up to one month post injection. PBMC are analyzed. Activated NK CD56+ CD3- Cord blood cells were transduced at MOI 50 and reactivated prior to injection into NOG-hL15 mice. Premium quality lentiviral vectors can transduce more than 65% of hNK cells, without toxicity and ensuring phenotype and cytotoxic activity.
Data kindly provided by Florence Borot, Mukherjee Lab - Columbia University, ICRC
Gene addition
Gene addition involves the introduction of a new gene or a gene corrected from unwanted mutations in order to target a specific aspect of a disease pathway. Such gene transfer therapeutic strategies can be used in complex or rare diseases, or in an infectious context as well, to target a microorganism through a specific antibody encoded by the gene transferred.
Flash therapeutics’ integrative lentiviral vectors are successfully used for gene addition approaches, in order to gene modify either the whole hematopoietic compartment or only T cells.
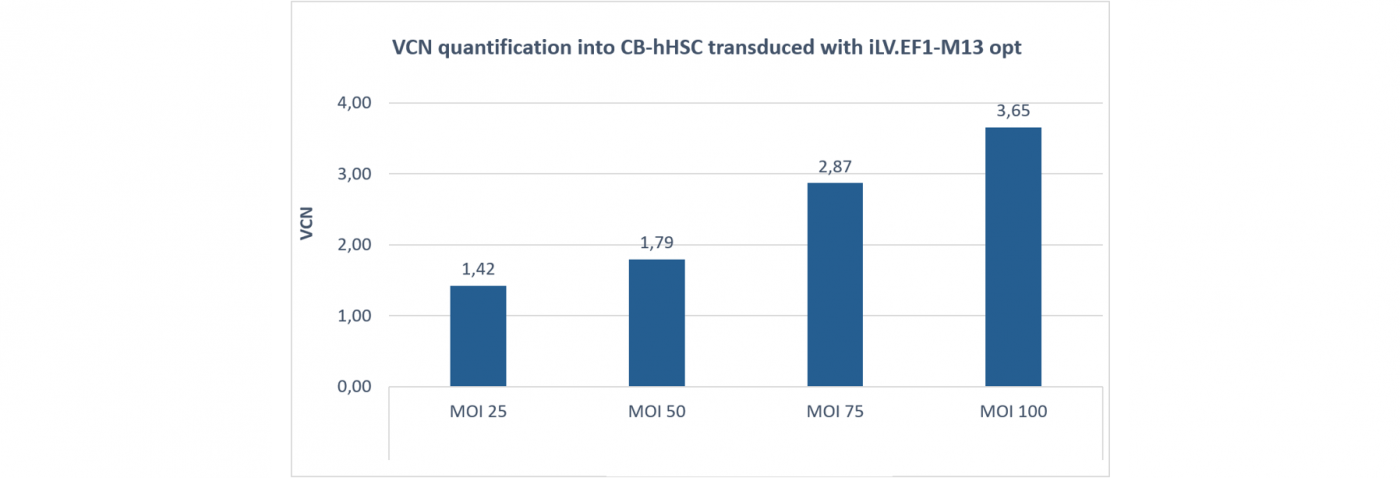
Gene addition VCN (Vector Copy Number) analysis obtained from human cord-blood derived HSCs transduced with increased doses of iLV expressing a gene of interest selected to treat a rare hematologic disease.
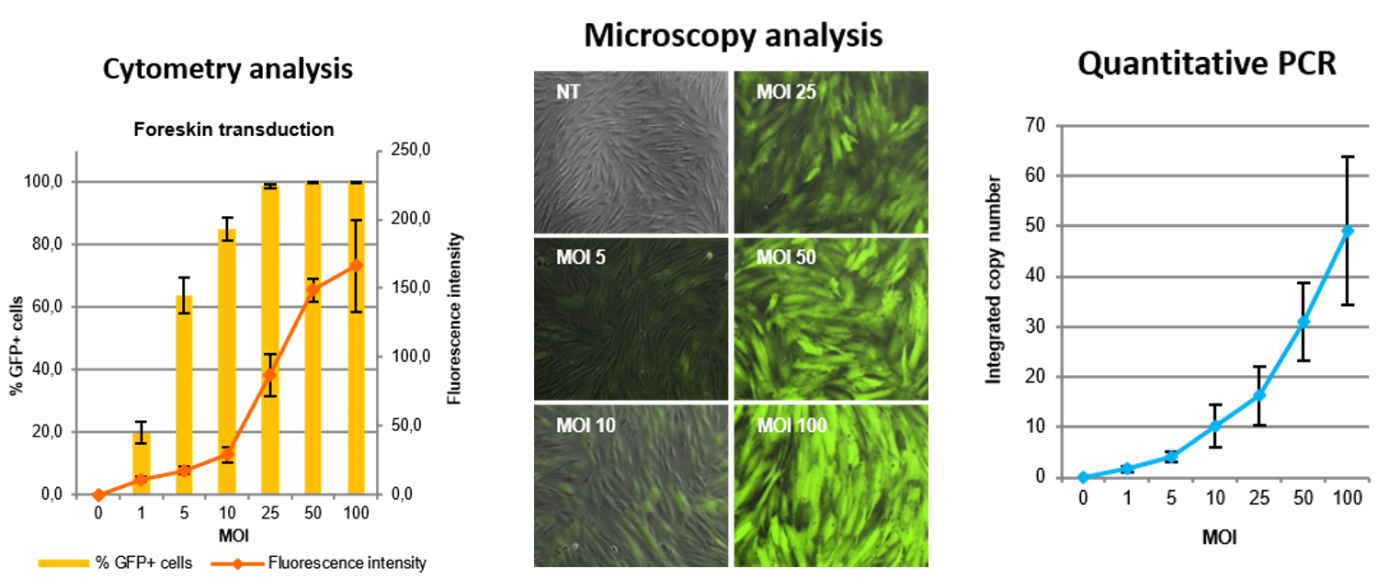
Stable and controlled quantitative expression for cell lines generation
In classical transfection approaches, the challenge is to ensure an appropriate and controlled expression level in target cells for any transgene. Flash Therapeutics’ lentiviral vectors offers the opportunity to observe a quantitative effect linked to a differential gene expression level, by using a controlled Multiplicity of Infection (MOI).
Being able to fully characterize expression rate and the number of copies in cells is essential to design robust screening assays.
Example of complete dose response analyzes on Forskin cells transduced with various MOI of GFP-expressing iLV:
Innovation pipeline
We are committed to constantly innovating in order to improve the properties of our vectors and make them the best tool for your project. Our team of experts works on:
- Developing packaging cell lines
- Modifying particles pseudotype to target specific cell types
- Optimizing production protocols to ensure any cassette, even very complex polycistronic ones can produce at a high titer, with a high infectivity.